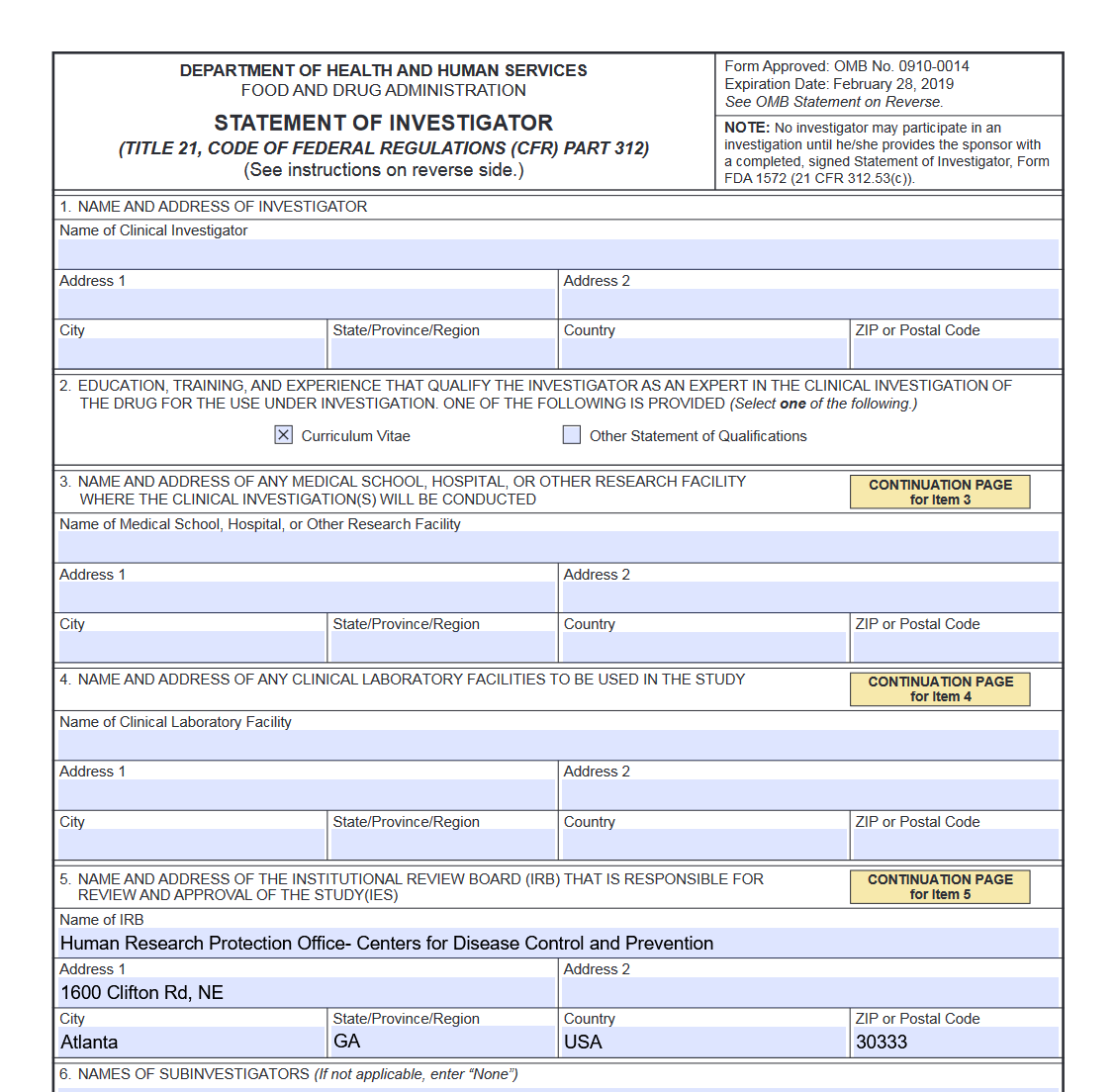
1572 Form Fda - For other fda forms, visit the fda forms page. What is the form fda 1572 (statement of investigator)? This fda page provides the form fda 1572, statement of investigator, for use in clinical trials and related research. The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain information to the sponsor and. The statement of investigator (form fda 1572) is a form that is required to be. This page provides links to commonly used clinical trial forms relevant to clinical trials.
The statement of investigator (form fda 1572) is a form that is required to be. This fda page provides the form fda 1572, statement of investigator, for use in clinical trials and related research. What is the form fda 1572 (statement of investigator)? The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain information to the sponsor and. This page provides links to commonly used clinical trial forms relevant to clinical trials. For other fda forms, visit the fda forms page.
For other fda forms, visit the fda forms page. The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain information to the sponsor and. The statement of investigator (form fda 1572) is a form that is required to be. What is the form fda 1572 (statement of investigator)? This page provides links to commonly used clinical trial forms relevant to clinical trials. This fda page provides the form fda 1572, statement of investigator, for use in clinical trials and related research.
Fda 1572 Template
This fda page provides the form fda 1572, statement of investigator, for use in clinical trials and related research. This page provides links to commonly used clinical trial forms relevant to clinical trials. The statement of investigator (form fda 1572) is a form that is required to be. The statement of investigator, form fda 1572 (1572), is an agreement signed.
Orientation for New Clinical Research PERSONNEL Module 2 ppt download
What is the form fda 1572 (statement of investigator)? This fda page provides the form fda 1572, statement of investigator, for use in clinical trials and related research. For other fda forms, visit the fda forms page. This page provides links to commonly used clinical trial forms relevant to clinical trials. The statement of investigator, form fda 1572 (1572), is.
PPT Regulatory Documentation Best Practices for Essential Clinical
For other fda forms, visit the fda forms page. The statement of investigator (form fda 1572) is a form that is required to be. This fda page provides the form fda 1572, statement of investigator, for use in clinical trials and related research. The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide.
PPT IND Basics in OCTGT PowerPoint Presentation, free download ID
The statement of investigator (form fda 1572) is a form that is required to be. This page provides links to commonly used clinical trial forms relevant to clinical trials. The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain information to the sponsor and. This fda page provides the form fda 1572,.
FDA_1572 Institutional Review Board Health Sciences
This fda page provides the form fda 1572, statement of investigator, for use in clinical trials and related research. The statement of investigator (form fda 1572) is a form that is required to be. What is the form fda 1572 (statement of investigator)? The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide.
InvestigatorStatement FDA 1572 Centers for Doc Template
The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain information to the sponsor and. This fda page provides the form fda 1572, statement of investigator, for use in clinical trials and related research. This page provides links to commonly used clinical trial forms relevant to clinical trials. The statement of investigator.
Fda 1572 Form 2023 Printable Forms Free Online
The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain information to the sponsor and. This page provides links to commonly used clinical trial forms relevant to clinical trials. What is the form fda 1572 (statement of investigator)? The statement of investigator (form fda 1572) is a form that is required to.
Form FDA 1572 Statement of Investigator Free Download
What is the form fda 1572 (statement of investigator)? The statement of investigator (form fda 1572) is a form that is required to be. This fda page provides the form fda 1572, statement of investigator, for use in clinical trials and related research. For other fda forms, visit the fda forms page. The statement of investigator, form fda 1572 (1572),.
IND Filing, Timelines, Paperwork and Reports September 3, 2010 Kate
This fda page provides the form fda 1572, statement of investigator, for use in clinical trials and related research. What is the form fda 1572 (statement of investigator)? For other fda forms, visit the fda forms page. The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain information to the sponsor and..
PPT Clinical Investigator Responsibilities Regulations and
This page provides links to commonly used clinical trial forms relevant to clinical trials. For other fda forms, visit the fda forms page. The statement of investigator (form fda 1572) is a form that is required to be. The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain information to the sponsor.
This Page Provides Links To Commonly Used Clinical Trial Forms Relevant To Clinical Trials.
This fda page provides the form fda 1572, statement of investigator, for use in clinical trials and related research. What is the form fda 1572 (statement of investigator)? For other fda forms, visit the fda forms page. The statement of investigator, form fda 1572 (1572), is an agreement signed by the investigator to provide certain information to the sponsor and.

.jpg)