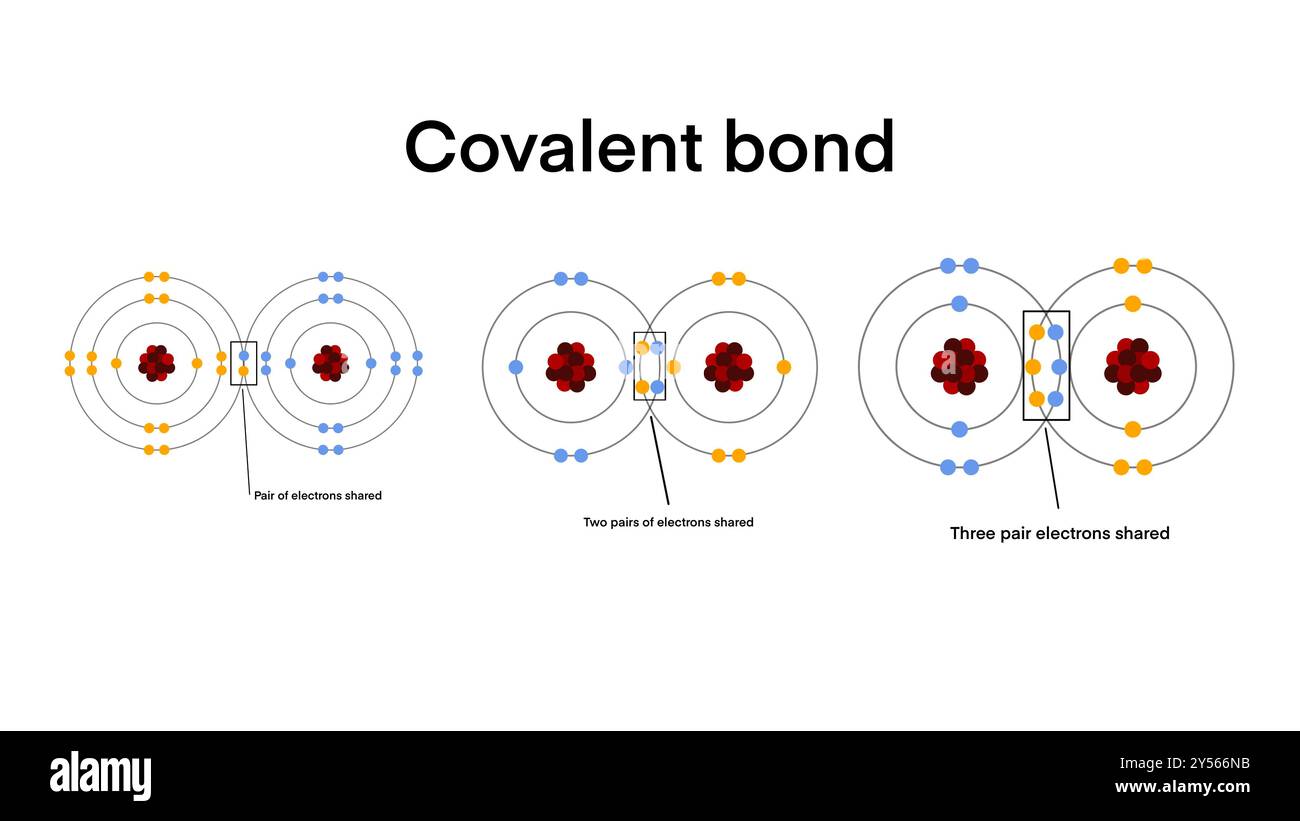
Bond Formed When Electrons Are Shared Between Atoms - Heterolytic bond breaking is also known as heterolysis or heterolytic fission or ionic fission. In this bond, both shared electrons are donated by the same atom. It is defined as breaking of. The bond formed between carbon and nitrogen has to be classified as nonpolar covalent, polar covalent or ionic. What is heterolytic bond breaking? 1 chemical foundations 2 atoms, molecules, and ions 3 stoichiometry 4 types of chemical reactions and solution stoichiometry 5 gases 6. A covalent bond is formed by mutual sharing of two electrons between the atoms, each atom giving one electron for sharing.
It is defined as breaking of. Heterolytic bond breaking is also known as heterolysis or heterolytic fission or ionic fission. What is heterolytic bond breaking? In this bond, both shared electrons are donated by the same atom. A covalent bond is formed by mutual sharing of two electrons between the atoms, each atom giving one electron for sharing. The bond formed between carbon and nitrogen has to be classified as nonpolar covalent, polar covalent or ionic. 1 chemical foundations 2 atoms, molecules, and ions 3 stoichiometry 4 types of chemical reactions and solution stoichiometry 5 gases 6.
A covalent bond is formed by mutual sharing of two electrons between the atoms, each atom giving one electron for sharing. Heterolytic bond breaking is also known as heterolysis or heterolytic fission or ionic fission. It is defined as breaking of. 1 chemical foundations 2 atoms, molecules, and ions 3 stoichiometry 4 types of chemical reactions and solution stoichiometry 5 gases 6. What is heterolytic bond breaking? The bond formed between carbon and nitrogen has to be classified as nonpolar covalent, polar covalent or ionic. In this bond, both shared electrons are donated by the same atom.
covalent bond is a chemical bond that involves the sharing of electrons
In this bond, both shared electrons are donated by the same atom. It is defined as breaking of. The bond formed between carbon and nitrogen has to be classified as nonpolar covalent, polar covalent or ionic. Heterolytic bond breaking is also known as heterolysis or heterolytic fission or ionic fission. What is heterolytic bond breaking?
What are Chemical bonds? Chemical bonds are formed between atoms when
What is heterolytic bond breaking? A covalent bond is formed by mutual sharing of two electrons between the atoms, each atom giving one electron for sharing. The bond formed between carbon and nitrogen has to be classified as nonpolar covalent, polar covalent or ionic. Heterolytic bond breaking is also known as heterolysis or heterolytic fission or ionic fission. 1 chemical.
Chapter 2 Atoms and Bonding ppt download
In this bond, both shared electrons are donated by the same atom. Heterolytic bond breaking is also known as heterolysis or heterolytic fission or ionic fission. A covalent bond is formed by mutual sharing of two electrons between the atoms, each atom giving one electron for sharing. The bond formed between carbon and nitrogen has to be classified as nonpolar.
The Chemistry of Biology ppt download
It is defined as breaking of. The bond formed between carbon and nitrogen has to be classified as nonpolar covalent, polar covalent or ionic. A covalent bond is formed by mutual sharing of two electrons between the atoms, each atom giving one electron for sharing. In this bond, both shared electrons are donated by the same atom. 1 chemical foundations.
Biochemistry. ppt download
Heterolytic bond breaking is also known as heterolysis or heterolytic fission or ionic fission. The bond formed between carbon and nitrogen has to be classified as nonpolar covalent, polar covalent or ionic. A covalent bond is formed by mutual sharing of two electrons between the atoms, each atom giving one electron for sharing. 1 chemical foundations 2 atoms, molecules, and.
GOOD MORNING!! Lets do a QW!! ppt download
A covalent bond is formed by mutual sharing of two electrons between the atoms, each atom giving one electron for sharing. What is heterolytic bond breaking? The bond formed between carbon and nitrogen has to be classified as nonpolar covalent, polar covalent or ionic. In this bond, both shared electrons are donated by the same atom. 1 chemical foundations 2.
Chemistry Review ppt download
What is heterolytic bond breaking? Heterolytic bond breaking is also known as heterolysis or heterolytic fission or ionic fission. It is defined as breaking of. In this bond, both shared electrons are donated by the same atom. The bond formed between carbon and nitrogen has to be classified as nonpolar covalent, polar covalent or ionic.
Electron Configurations & The Periodic Table
Heterolytic bond breaking is also known as heterolysis or heterolytic fission or ionic fission. What is heterolytic bond breaking? The bond formed between carbon and nitrogen has to be classified as nonpolar covalent, polar covalent or ionic. A covalent bond is formed by mutual sharing of two electrons between the atoms, each atom giving one electron for sharing. 1 chemical.
Covalent Bonding (Biology) — Definition & Role Expii
What is heterolytic bond breaking? A covalent bond is formed by mutual sharing of two electrons between the atoms, each atom giving one electron for sharing. Heterolytic bond breaking is also known as heterolysis or heterolytic fission or ionic fission. 1 chemical foundations 2 atoms, molecules, and ions 3 stoichiometry 4 types of chemical reactions and solution stoichiometry 5 gases.
Chapter 2 Chemistry of Life ppt download
What is heterolytic bond breaking? A covalent bond is formed by mutual sharing of two electrons between the atoms, each atom giving one electron for sharing. In this bond, both shared electrons are donated by the same atom. 1 chemical foundations 2 atoms, molecules, and ions 3 stoichiometry 4 types of chemical reactions and solution stoichiometry 5 gases 6. Heterolytic.
A Covalent Bond Is Formed By Mutual Sharing Of Two Electrons Between The Atoms, Each Atom Giving One Electron For Sharing.
What is heterolytic bond breaking? The bond formed between carbon and nitrogen has to be classified as nonpolar covalent, polar covalent or ionic. Heterolytic bond breaking is also known as heterolysis or heterolytic fission or ionic fission. It is defined as breaking of.
In This Bond, Both Shared Electrons Are Donated By The Same Atom.
1 chemical foundations 2 atoms, molecules, and ions 3 stoichiometry 4 types of chemical reactions and solution stoichiometry 5 gases 6.