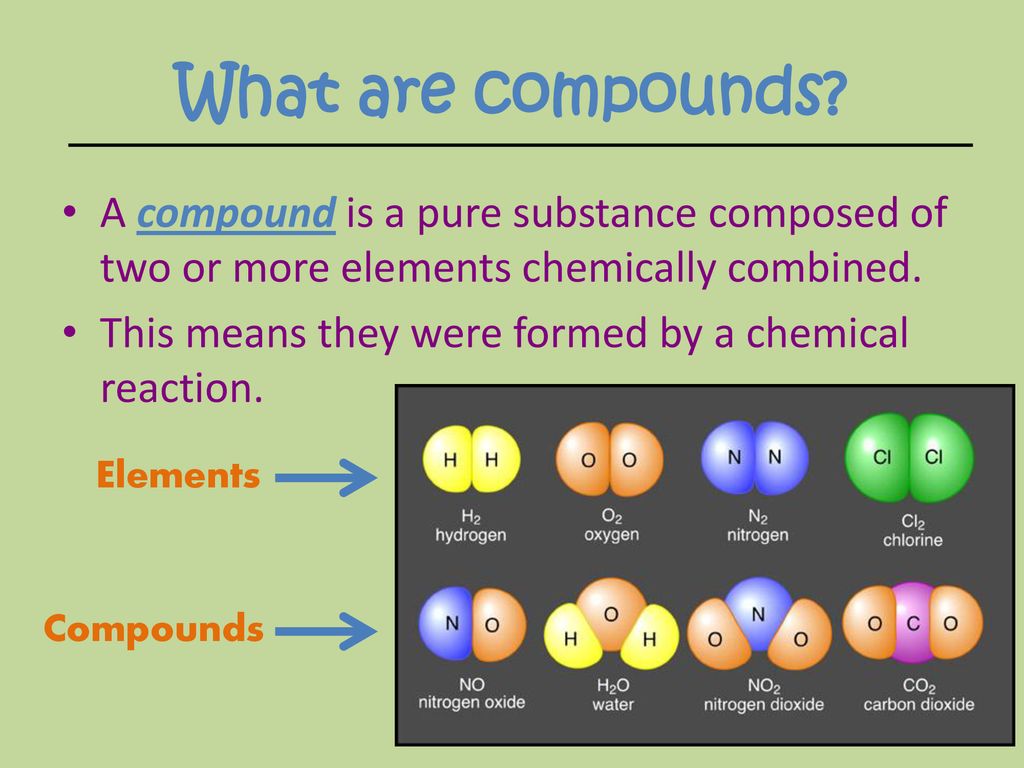
Compounds Are Formed - Lets take the ionic formula for calcium chloride, cacl_2. Compounds that contain carbon, hydrogen, and oxygen are organic. Calcium is an alkaline earth metal in the second column of the periodic table. How do ionic compounds conduct electricity in water? Because water is a solvent that is capable of solvating cations, and to a lesser extents anions. Structural isomers are compounds of the same chemical formula but different connectivities. How do ionic compounds dissolve? Is it possible to distinguish between ionic and. Of course, not all ionic compounds are soluble in. In living things, organic compounds are often called biomolecules, but.
Lets take the ionic formula for calcium chloride, cacl_2. Compounds that contain carbon, hydrogen, and oxygen are organic. How do ionic compounds conduct electricity in water? Calcium is an alkaline earth metal in the second column of the periodic table. Structural isomers are compounds of the same chemical formula but different connectivities. Of course, not all ionic compounds are soluble in. How do ionic compounds dissolve? Because water is a solvent that is capable of solvating cations, and to a lesser extents anions. Is it possible to distinguish between ionic and. In living things, organic compounds are often called biomolecules, but.
How do ionic compounds conduct electricity in water? Structural isomers are compounds of the same chemical formula but different connectivities. In living things, organic compounds are often called biomolecules, but. Calcium is an alkaline earth metal in the second column of the periodic table. Lets take the ionic formula for calcium chloride, cacl_2. Compounds that contain carbon, hydrogen, and oxygen are organic. How do ionic compounds dissolve? Is it possible to distinguish between ionic and. Of course, not all ionic compounds are soluble in. Because water is a solvent that is capable of solvating cations, and to a lesser extents anions.
Chemistry Introduction to and Classification of Matter ppt download
Because water is a solvent that is capable of solvating cations, and to a lesser extents anions. How do ionic compounds dissolve? How do ionic compounds conduct electricity in water? Is it possible to distinguish between ionic and. Structural isomers are compounds of the same chemical formula but different connectivities.
EARTH CHEMISTRY Matter, Elements, Compounds and Molecules ppt download
How do ionic compounds dissolve? Of course, not all ionic compounds are soluble in. Compounds that contain carbon, hydrogen, and oxygen are organic. Because water is a solvent that is capable of solvating cations, and to a lesser extents anions. Structural isomers are compounds of the same chemical formula but different connectivities.
The wonderful world of Chemistry ppt download
In living things, organic compounds are often called biomolecules, but. Compounds that contain carbon, hydrogen, and oxygen are organic. Is it possible to distinguish between ionic and. Lets take the ionic formula for calcium chloride, cacl_2. Structural isomers are compounds of the same chemical formula but different connectivities.
List Of Compounds
Of course, not all ionic compounds are soluble in. Lets take the ionic formula for calcium chloride, cacl_2. Structural isomers are compounds of the same chemical formula but different connectivities. Compounds that contain carbon, hydrogen, and oxygen are organic. Calcium is an alkaline earth metal in the second column of the periodic table.
Nomenclature Naming Chemicals ppt download
In living things, organic compounds are often called biomolecules, but. Because water is a solvent that is capable of solvating cations, and to a lesser extents anions. Calcium is an alkaline earth metal in the second column of the periodic table. Of course, not all ionic compounds are soluble in. Structural isomers are compounds of the same chemical formula but.
The Chemistry of Life Chapter ppt video online download
Structural isomers are compounds of the same chemical formula but different connectivities. Because water is a solvent that is capable of solvating cations, and to a lesser extents anions. Of course, not all ionic compounds are soluble in. Is it possible to distinguish between ionic and. How do ionic compounds conduct electricity in water?
Aim How do we classify matter? ppt download
How do ionic compounds conduct electricity in water? Because water is a solvent that is capable of solvating cations, and to a lesser extents anions. Of course, not all ionic compounds are soluble in. Lets take the ionic formula for calcium chloride, cacl_2. In living things, organic compounds are often called biomolecules, but.
Substances and Mixtures ppt download
How do ionic compounds conduct electricity in water? Compounds that contain carbon, hydrogen, and oxygen are organic. Is it possible to distinguish between ionic and. Lets take the ionic formula for calcium chloride, cacl_2. Because water is a solvent that is capable of solvating cations, and to a lesser extents anions.
Elements, Compounds & Mixtures! Mr. Coffey. ppt download
Lets take the ionic formula for calcium chloride, cacl_2. Is it possible to distinguish between ionic and. Compounds that contain carbon, hydrogen, and oxygen are organic. How do ionic compounds conduct electricity in water? Calcium is an alkaline earth metal in the second column of the periodic table.
Minerals Chapter 3 Pearson. ppt download
Structural isomers are compounds of the same chemical formula but different connectivities. In living things, organic compounds are often called biomolecules, but. Calcium is an alkaline earth metal in the second column of the periodic table. Lets take the ionic formula for calcium chloride, cacl_2. How do ionic compounds dissolve?
Structural Isomers Are Compounds Of The Same Chemical Formula But Different Connectivities.
Compounds that contain carbon, hydrogen, and oxygen are organic. Of course, not all ionic compounds are soluble in. How do ionic compounds conduct electricity in water? In living things, organic compounds are often called biomolecules, but.
Lets Take The Ionic Formula For Calcium Chloride, Cacl_2.
Is it possible to distinguish between ionic and. Because water is a solvent that is capable of solvating cations, and to a lesser extents anions. How do ionic compounds dissolve? Calcium is an alkaline earth metal in the second column of the periodic table.