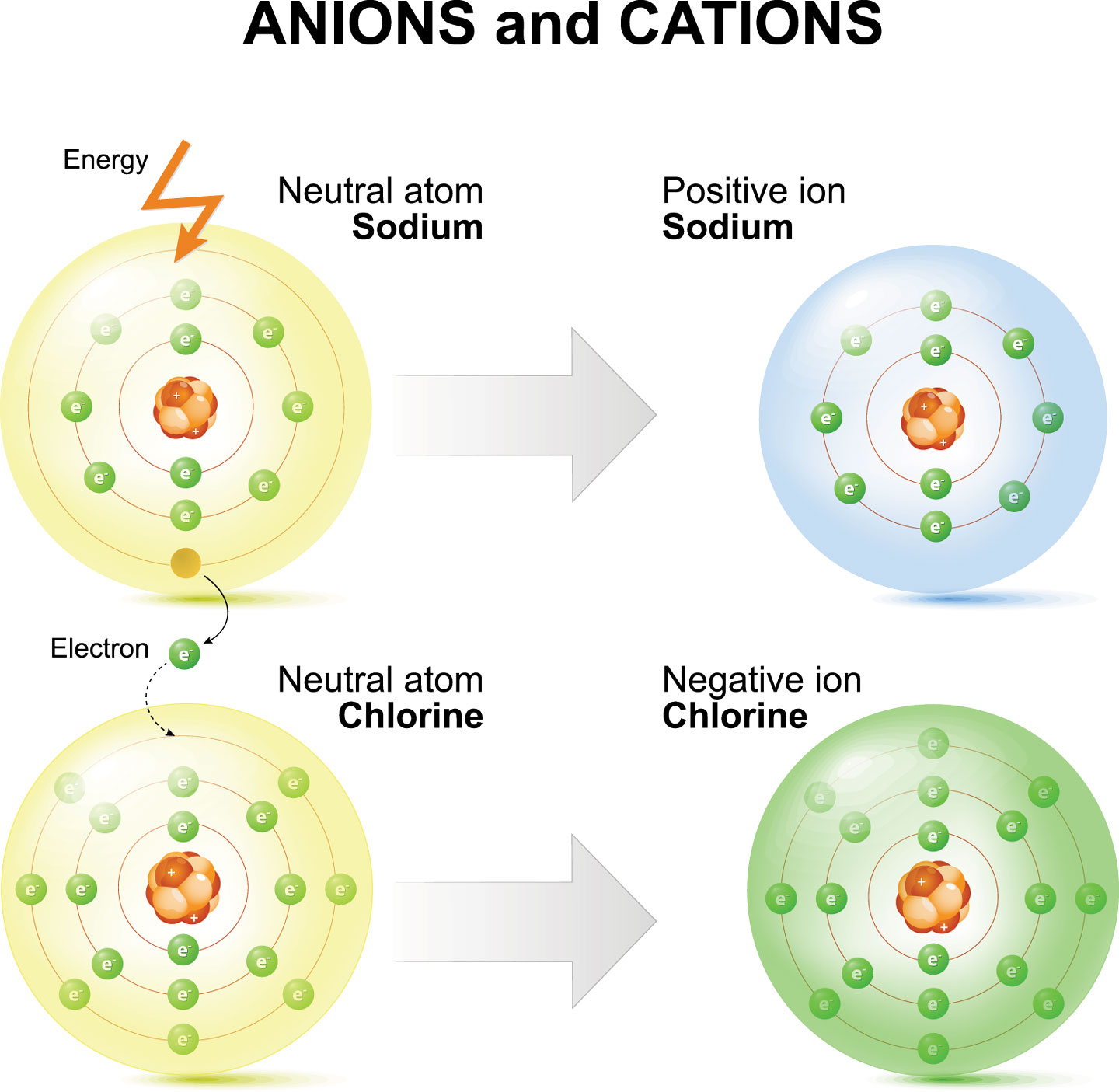
How Does A Cation Form - Boron is a metalloid element with the symbol b. Fluorine does not form cations, or any compound, complex ion, or coordinate. Is boron a cation or an anion? In chemical compounds, boron tends to form covalent bonds. Fluorine is a neutral atom, though fluoride is an anion. The lead ion is a cation, meaning that it has a positive charge. Argon typically does not form ions because it has a full outer electron shell. This stable electron configuration prevents argon from. However, there are multiple lead cations, each with a different charge. Scandium is a cation because it tends to lose electrons to form a positive charge.
In chemical compounds, boron tends to form covalent bonds. This stable electron configuration prevents argon from. However, there are multiple lead cations, each with a different charge. Argon typically does not form ions because it has a full outer electron shell. Is boron a cation or an anion? The lead ion is a cation, meaning that it has a positive charge. Fluorine is a neutral atom, though fluoride is an anion. Fluorine does not form cations, or any compound, complex ion, or coordinate. Boron is a metalloid element with the symbol b. Scandium is a cation because it tends to lose electrons to form a positive charge.
Argon typically does not form ions because it has a full outer electron shell. Is boron a cation or an anion? Fluorine does not form cations, or any compound, complex ion, or coordinate. However, there are multiple lead cations, each with a different charge. Scandium is a cation because it tends to lose electrons to form a positive charge. The lead ion is a cation, meaning that it has a positive charge. Boron is a metalloid element with the symbol b. In chemical compounds, boron tends to form covalent bonds. Fluorine is a neutral atom, though fluoride is an anion. This stable electron configuration prevents argon from.
Valence elecrtrons and chemical properties ppt download
The lead ion is a cation, meaning that it has a positive charge. Boron is a metalloid element with the symbol b. Argon typically does not form ions because it has a full outer electron shell. Fluorine is a neutral atom, though fluoride is an anion. Fluorine does not form cations, or any compound, complex ion, or coordinate.
Unit 5 Ionic Bonding & Nomenclature ppt download
Boron is a metalloid element with the symbol b. The lead ion is a cation, meaning that it has a positive charge. Fluorine is a neutral atom, though fluoride is an anion. Is boron a cation or an anion? In chemical compounds, boron tends to form covalent bonds.
Chapter 6B Chemical Bonding ppt download
Fluorine is a neutral atom, though fluoride is an anion. Scandium is a cation because it tends to lose electrons to form a positive charge. However, there are multiple lead cations, each with a different charge. Boron is a metalloid element with the symbol b. Argon typically does not form ions because it has a full outer electron shell.
Chapter 6 Bonding. ppt download
The lead ion is a cation, meaning that it has a positive charge. Boron is a metalloid element with the symbol b. This stable electron configuration prevents argon from. However, there are multiple lead cations, each with a different charge. Is boron a cation or an anion?
Ions. ppt download
Is boron a cation or an anion? However, there are multiple lead cations, each with a different charge. This stable electron configuration prevents argon from. Argon typically does not form ions because it has a full outer electron shell. Fluorine is a neutral atom, though fluoride is an anion.
Structure & Bonding. ppt download
The lead ion is a cation, meaning that it has a positive charge. Boron is a metalloid element with the symbol b. Argon typically does not form ions because it has a full outer electron shell. However, there are multiple lead cations, each with a different charge. This stable electron configuration prevents argon from.
Cations and Anions Definitions, Examples, and Differences
Fluorine is a neutral atom, though fluoride is an anion. This stable electron configuration prevents argon from. The lead ion is a cation, meaning that it has a positive charge. Is boron a cation or an anion? Boron is a metalloid element with the symbol b.
Chapters 7 and 8 Bonding. ppt download
In chemical compounds, boron tends to form covalent bonds. The lead ion is a cation, meaning that it has a positive charge. This stable electron configuration prevents argon from. Is boron a cation or an anion? Scandium is a cation because it tends to lose electrons to form a positive charge.
Chemistry ppt download
The lead ion is a cation, meaning that it has a positive charge. In chemical compounds, boron tends to form covalent bonds. Scandium is a cation because it tends to lose electrons to form a positive charge. Boron is a metalloid element with the symbol b. Is boron a cation or an anion?
Explainer Ions and radicals in our world Science News for Students
Is boron a cation or an anion? Fluorine is a neutral atom, though fluoride is an anion. Fluorine does not form cations, or any compound, complex ion, or coordinate. Scandium is a cation because it tends to lose electrons to form a positive charge. Boron is a metalloid element with the symbol b.
Argon Typically Does Not Form Ions Because It Has A Full Outer Electron Shell.
The lead ion is a cation, meaning that it has a positive charge. Is boron a cation or an anion? However, there are multiple lead cations, each with a different charge. Scandium is a cation because it tends to lose electrons to form a positive charge.
In Chemical Compounds, Boron Tends To Form Covalent Bonds.
Fluorine does not form cations, or any compound, complex ion, or coordinate. Fluorine is a neutral atom, though fluoride is an anion. Boron is a metalloid element with the symbol b. This stable electron configuration prevents argon from.


:+This+loss+of+electrons+is+called+oxidation..jpg)