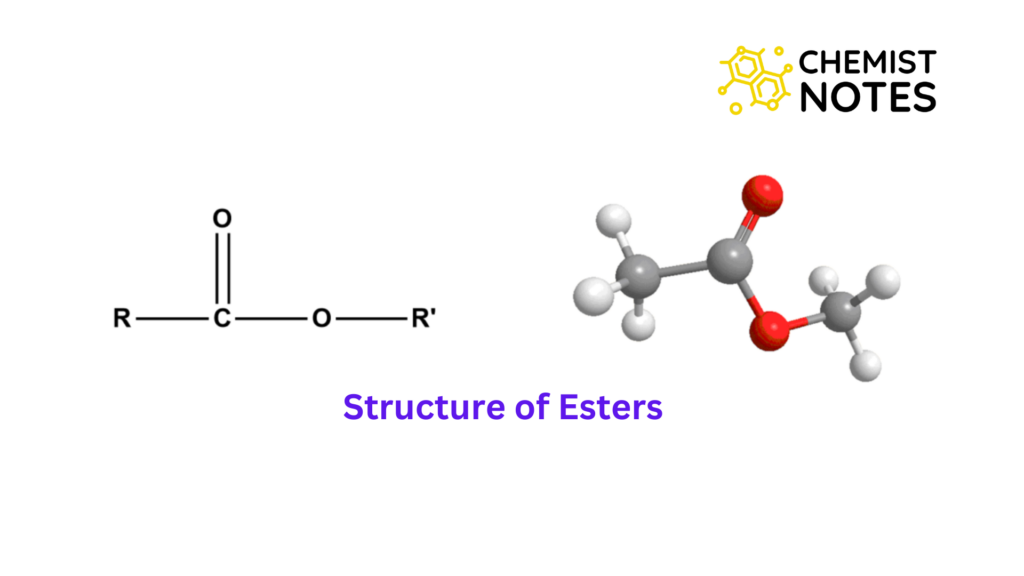
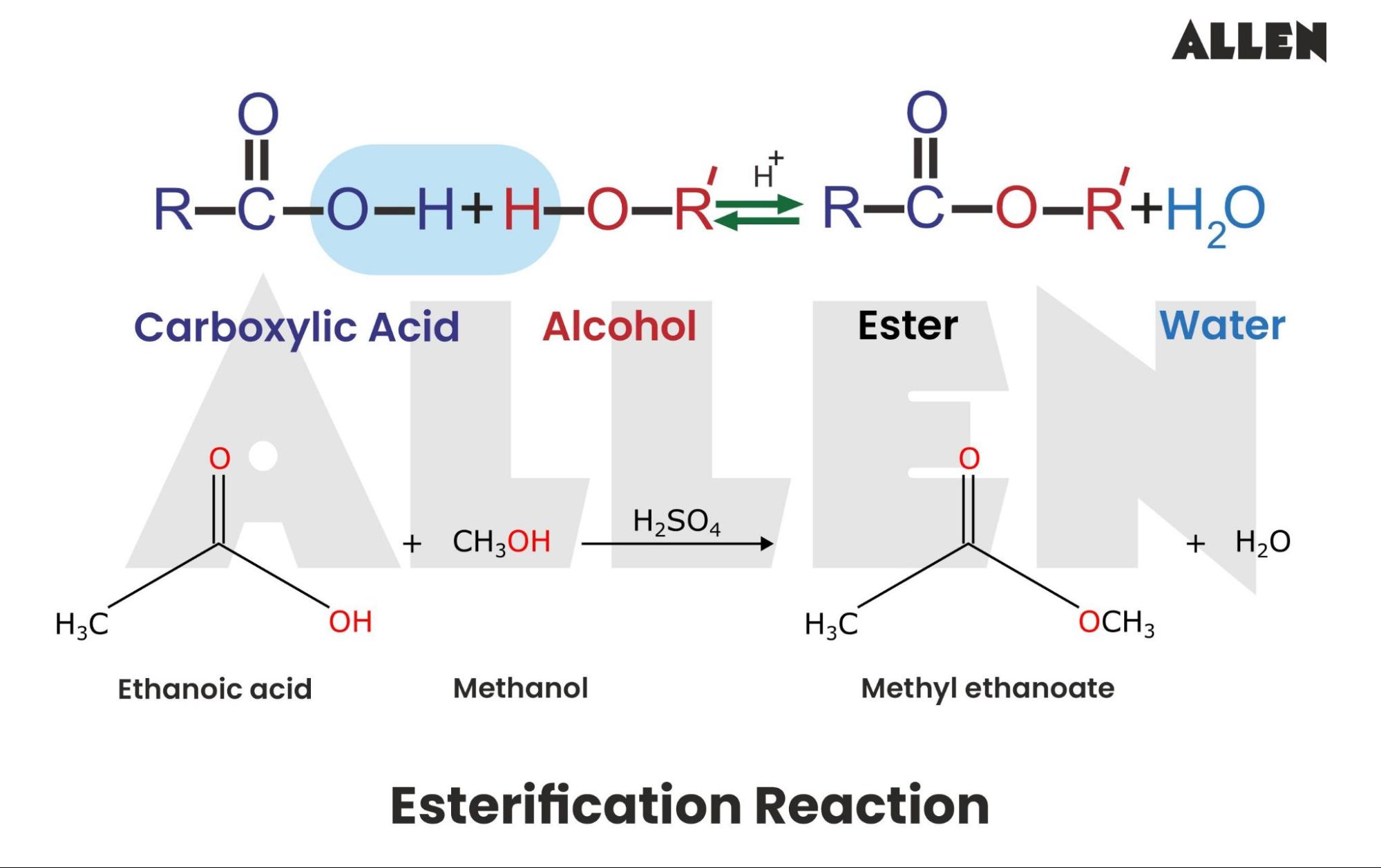
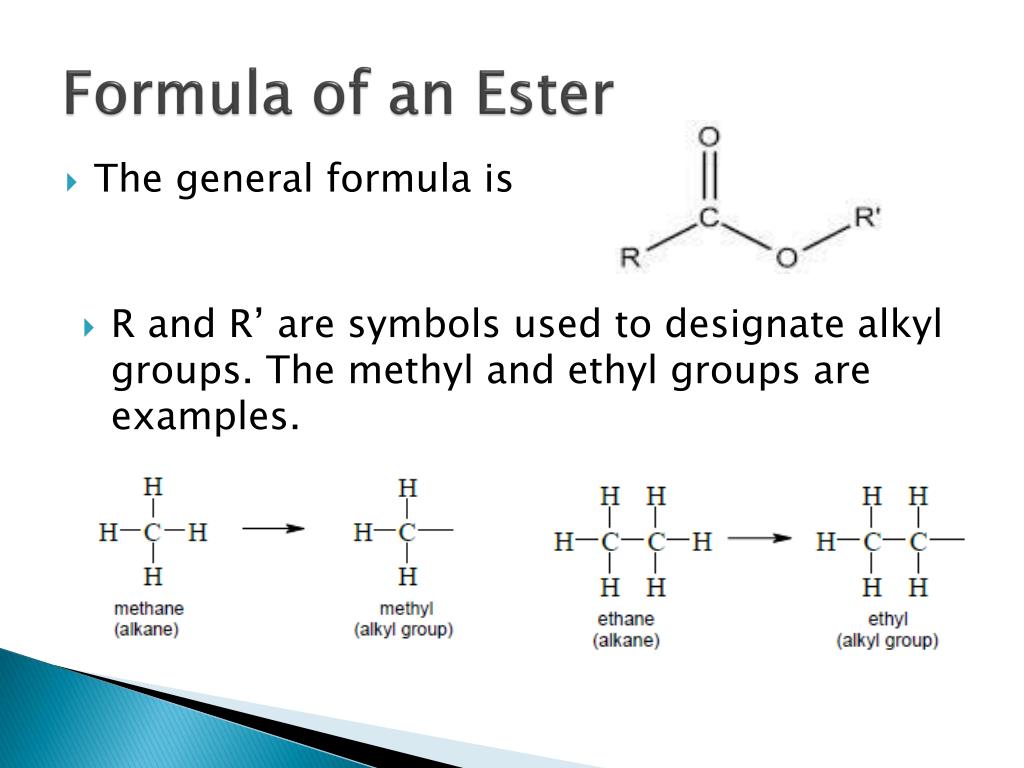
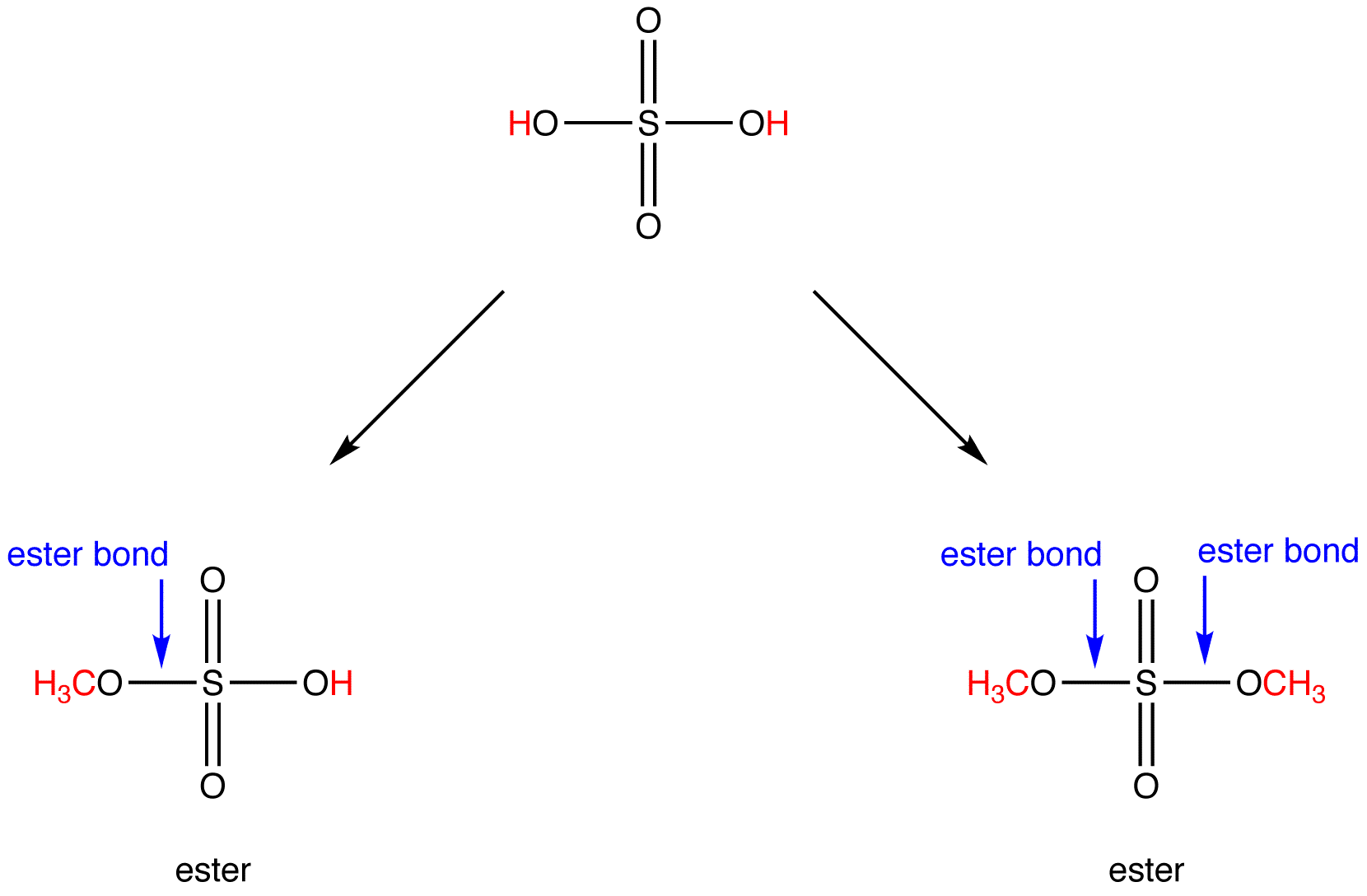
How Ester Is Formed - Ester, any of a class of organic compounds that react with water to produce alcohols and organic or inorganic acids. Esters are derived from the condensation. An ester is an organic compound where the hydrogen in the compound's carboxyl group is replaced with a hydrocarbon group. Key takeaway an ester has an or group attached to the carbon atom of a carbonyl group. In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (h) of at least one acidic hydroxyl. The general structure of an ester is rcoor', where r and r' represent alkyl or aryl groups.
Key takeaway an ester has an or group attached to the carbon atom of a carbonyl group. The general structure of an ester is rcoor', where r and r' represent alkyl or aryl groups. Ester, any of a class of organic compounds that react with water to produce alcohols and organic or inorganic acids. Esters are derived from the condensation. An ester is an organic compound where the hydrogen in the compound's carboxyl group is replaced with a hydrocarbon group. In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (h) of at least one acidic hydroxyl.
Key takeaway an ester has an or group attached to the carbon atom of a carbonyl group. The general structure of an ester is rcoor', where r and r' represent alkyl or aryl groups. Ester, any of a class of organic compounds that react with water to produce alcohols and organic or inorganic acids. Esters are derived from the condensation. An ester is an organic compound where the hydrogen in the compound's carboxyl group is replaced with a hydrocarbon group. In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (h) of at least one acidic hydroxyl.
Carboxylic acids. ppt download
An ester is an organic compound where the hydrogen in the compound's carboxyl group is replaced with a hydrocarbon group. In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (h) of at least one acidic hydroxyl. Esters are derived from the condensation. The general structure of an ester is.
Q What are living things made of? A We are what we eat. ppt download
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (h) of at least one acidic hydroxyl. Ester, any of a class of organic compounds that react with water to produce alcohols and organic or inorganic acids. Esters are derived from the condensation. The general structure of an ester is.
Organic Preparations Question 2 ppt download
Esters are derived from the condensation. Key takeaway an ester has an or group attached to the carbon atom of a carbonyl group. An ester is an organic compound where the hydrogen in the compound's carboxyl group is replaced with a hydrocarbon group. The general structure of an ester is rcoor', where r and r' represent alkyl or aryl groups..
[Carbon] Ethanoic acid Formation, Properties, Uses [with Reactions]
The general structure of an ester is rcoor', where r and r' represent alkyl or aryl groups. Ester, any of a class of organic compounds that react with water to produce alcohols and organic or inorganic acids. Esters are derived from the condensation. In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which.
Esters Definition, Nomenclature, Preparation, Properties, and
Esters are derived from the condensation. In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (h) of at least one acidic hydroxyl. Key takeaway an ester has an or group attached to the carbon atom of a carbonyl group. An ester is an organic compound where the hydrogen in.
Ester Structural Formula
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (h) of at least one acidic hydroxyl. Esters are derived from the condensation. The general structure of an ester is rcoor', where r and r' represent alkyl or aryl groups. Key takeaway an ester has an or group attached to.
Chapter 9 Carbon Chemistry ppt download
Esters are derived from the condensation. The general structure of an ester is rcoor', where r and r' represent alkyl or aryl groups. Key takeaway an ester has an or group attached to the carbon atom of a carbonyl group. An ester is an organic compound where the hydrogen in the compound's carboxyl group is replaced with a hydrocarbon group..
Esterification Revisiting Glycerol Esterification With Acetic Acid
An ester is an organic compound where the hydrogen in the compound's carboxyl group is replaced with a hydrocarbon group. Ester, any of a class of organic compounds that react with water to produce alcohols and organic or inorganic acids. The general structure of an ester is rcoor', where r and r' represent alkyl or aryl groups. Esters are derived.
PPT Esters PowerPoint Presentation, free download ID2192073
An ester is an organic compound where the hydrogen in the compound's carboxyl group is replaced with a hydrocarbon group. In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (h) of at least one acidic hydroxyl. Ester, any of a class of organic compounds that react with water to.
Ester Chemistry LibreTexts
Esters are derived from the condensation. The general structure of an ester is rcoor', where r and r' represent alkyl or aryl groups. Key takeaway an ester has an or group attached to the carbon atom of a carbonyl group. An ester is an organic compound where the hydrogen in the compound's carboxyl group is replaced with a hydrocarbon group..
Esters Are Derived From The Condensation.
An ester is an organic compound where the hydrogen in the compound's carboxyl group is replaced with a hydrocarbon group. Key takeaway an ester has an or group attached to the carbon atom of a carbonyl group. In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (h) of at least one acidic hydroxyl. The general structure of an ester is rcoor', where r and r' represent alkyl or aryl groups.



![[Carbon] Ethanoic acid Formation, Properties, Uses [with Reactions]](https://d1avenlh0i1xmr.cloudfront.net/a701667f-5d57-42e4-840d-8730fca08f85/esterfication-reaction---teachoo.jpg)