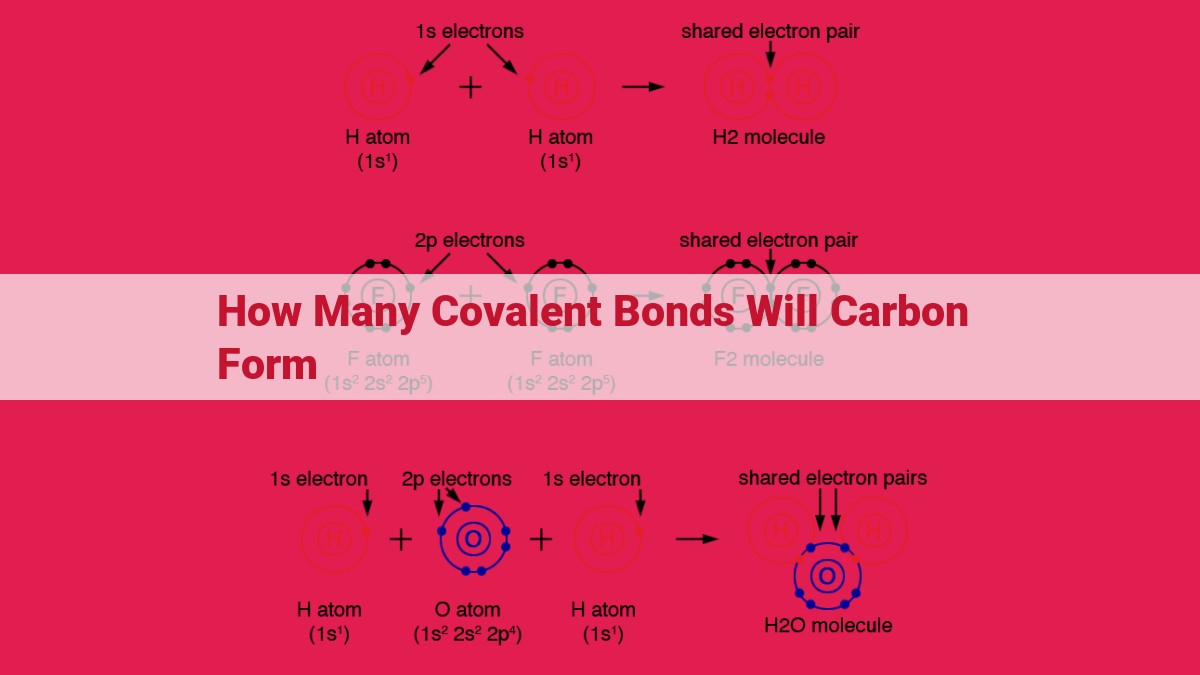
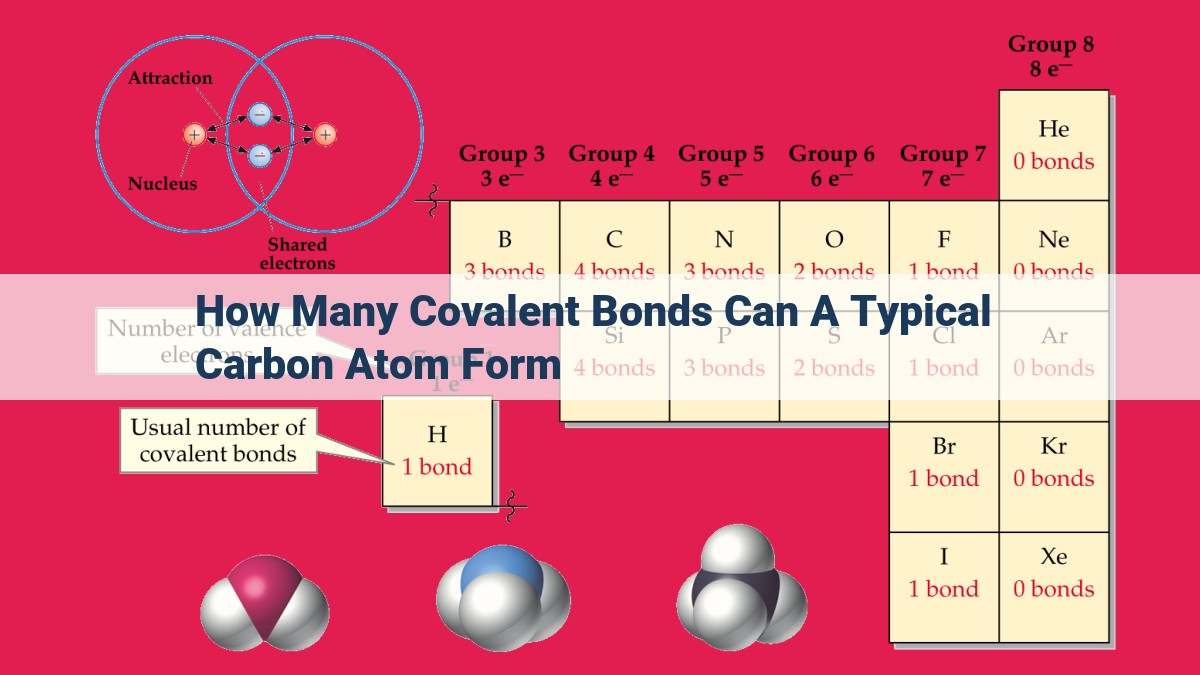
How Many Covalent Bonds Can Carbon Form - Carbon is a chemical element with the symbol c and atomic number 6. Since carbon has 4 valence electrons, it can form. This allows carbon to create various compounds, such. A carbon atom can form four covalent bonds due to having four valence electrons. Thus, a carbon atom can form a total of four covalent bonds with other atoms, as demonstrated in the methane molecule (ch4),. This allows carbon to share electrons with other atoms and reach the stable configuration of 8 electrons in its valence shell. It has 6 protons, 6 neutrons, and 6 electrons. Of these 6 electrons, 4. An atom can form covalent bonds by sharing its valence electrons with other atoms.
This allows carbon to share electrons with other atoms and reach the stable configuration of 8 electrons in its valence shell. A carbon atom can form four covalent bonds due to having four valence electrons. Since carbon has 4 valence electrons, it can form. An atom can form covalent bonds by sharing its valence electrons with other atoms. Thus, a carbon atom can form a total of four covalent bonds with other atoms, as demonstrated in the methane molecule (ch4),. This allows carbon to create various compounds, such. Of these 6 electrons, 4. It has 6 protons, 6 neutrons, and 6 electrons. Carbon is a chemical element with the symbol c and atomic number 6.
This allows carbon to share electrons with other atoms and reach the stable configuration of 8 electrons in its valence shell. Carbon is a chemical element with the symbol c and atomic number 6. It has 6 protons, 6 neutrons, and 6 electrons. Since carbon has 4 valence electrons, it can form. Thus, a carbon atom can form a total of four covalent bonds with other atoms, as demonstrated in the methane molecule (ch4),. This allows carbon to create various compounds, such. An atom can form covalent bonds by sharing its valence electrons with other atoms. Of these 6 electrons, 4. A carbon atom can form four covalent bonds due to having four valence electrons.
Part 2 Organic Chemistry (Carbon and Macromolecules) ppt download
This allows carbon to create various compounds, such. Thus, a carbon atom can form a total of four covalent bonds with other atoms, as demonstrated in the methane molecule (ch4),. Carbon is a chemical element with the symbol c and atomic number 6. It has 6 protons, 6 neutrons, and 6 electrons. Since carbon has 4 valence electrons, it can.
Unit 2 (Biochemistry) Notes, Part 1 Atomic And Molecular Structure
This allows carbon to share electrons with other atoms and reach the stable configuration of 8 electrons in its valence shell. Thus, a carbon atom can form a total of four covalent bonds with other atoms, as demonstrated in the methane molecule (ch4),. Of these 6 electrons, 4. This allows carbon to create various compounds, such. Since carbon has 4.
Carbon Compounds Section ppt download
A carbon atom can form four covalent bonds due to having four valence electrons. An atom can form covalent bonds by sharing its valence electrons with other atoms. Thus, a carbon atom can form a total of four covalent bonds with other atoms, as demonstrated in the methane molecule (ch4),. This allows carbon to share electrons with other atoms and.
MACROMOLECULES a.k.a. BioMolecules a.k.a. Organic Molecules ppt download
Since carbon has 4 valence electrons, it can form. Thus, a carbon atom can form a total of four covalent bonds with other atoms, as demonstrated in the methane molecule (ch4),. Of these 6 electrons, 4. It has 6 protons, 6 neutrons, and 6 electrons. Carbon is a chemical element with the symbol c and atomic number 6.
The Chemistry of Carbon = Chemistry of Life ppt download
Since carbon has 4 valence electrons, it can form. An atom can form covalent bonds by sharing its valence electrons with other atoms. Of these 6 electrons, 4. This allows carbon to share electrons with other atoms and reach the stable configuration of 8 electrons in its valence shell. A carbon atom can form four covalent bonds due to having.
PPT Organic Chemistry Functional Groups PowerPoint Presentation
It has 6 protons, 6 neutrons, and 6 electrons. Of these 6 electrons, 4. Since carbon has 4 valence electrons, it can form. An atom can form covalent bonds by sharing its valence electrons with other atoms. This allows carbon to share electrons with other atoms and reach the stable configuration of 8 electrons in its valence shell.
PPT Introduction to Carbon Chemistry PowerPoint Presentation, free
An atom can form covalent bonds by sharing its valence electrons with other atoms. Since carbon has 4 valence electrons, it can form. A carbon atom can form four covalent bonds due to having four valence electrons. Thus, a carbon atom can form a total of four covalent bonds with other atoms, as demonstrated in the methane molecule (ch4),. Carbon.
Mastering Carbon Bonds Understanding Hybridization and Covalent Bonding
This allows carbon to share electrons with other atoms and reach the stable configuration of 8 electrons in its valence shell. A carbon atom can form four covalent bonds due to having four valence electrons. An atom can form covalent bonds by sharing its valence electrons with other atoms. This allows carbon to create various compounds, such. Since carbon has.
[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds
Thus, a carbon atom can form a total of four covalent bonds with other atoms, as demonstrated in the methane molecule (ch4),. This allows carbon to create various compounds, such. This allows carbon to share electrons with other atoms and reach the stable configuration of 8 electrons in its valence shell. Of these 6 electrons, 4. Carbon is a chemical.
Understanding Carbon's Bonding Properties for Stable Molecular Formations
This allows carbon to create various compounds, such. An atom can form covalent bonds by sharing its valence electrons with other atoms. It has 6 protons, 6 neutrons, and 6 electrons. Since carbon has 4 valence electrons, it can form. This allows carbon to share electrons with other atoms and reach the stable configuration of 8 electrons in its valence.
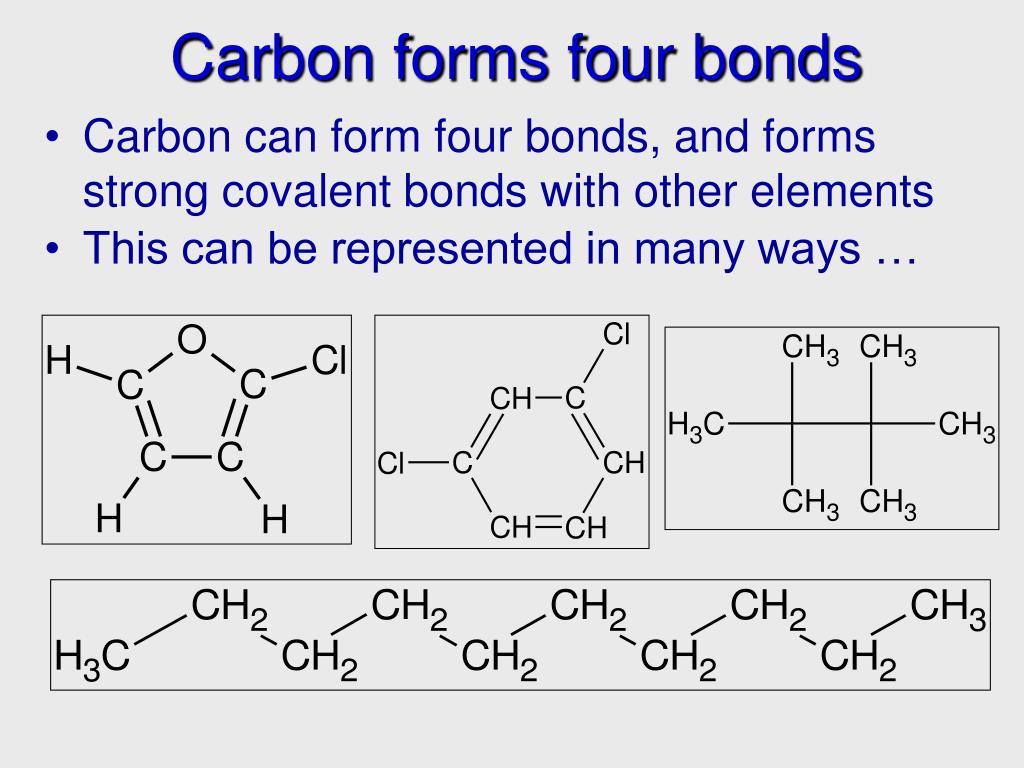
Thus, A Carbon Atom Can Form A Total Of Four Covalent Bonds With Other Atoms, As Demonstrated In The Methane Molecule (Ch4),.
This allows carbon to share electrons with other atoms and reach the stable configuration of 8 electrons in its valence shell. Since carbon has 4 valence electrons, it can form. Of these 6 electrons, 4. A carbon atom can form four covalent bonds due to having four valence electrons.
It Has 6 Protons, 6 Neutrons, And 6 Electrons.
Carbon is a chemical element with the symbol c and atomic number 6. This allows carbon to create various compounds, such. An atom can form covalent bonds by sharing its valence electrons with other atoms.






![[Class 10 Chemistry] Bonding in Carbon Atoms Covalent Bonds](https://d1avenlh0i1xmr.cloudfront.net/large/693c5ecd-56f0-42a1-aba9-2e5a5be31300/covalent-bonding-in-co2---teachoo.jpg)