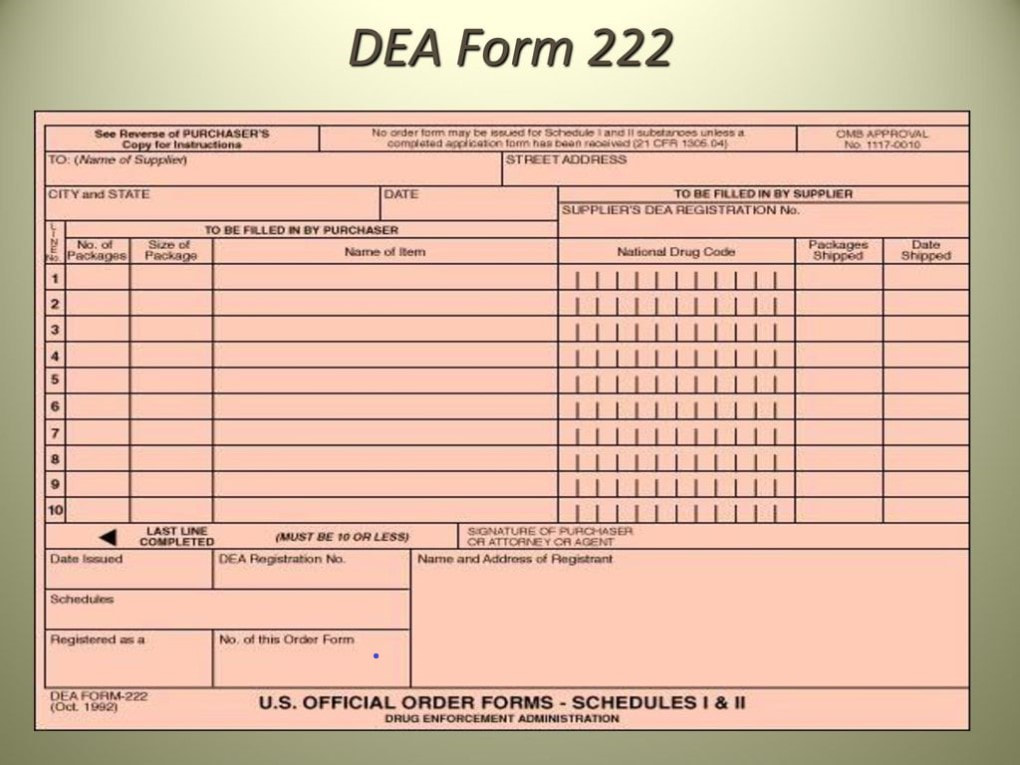
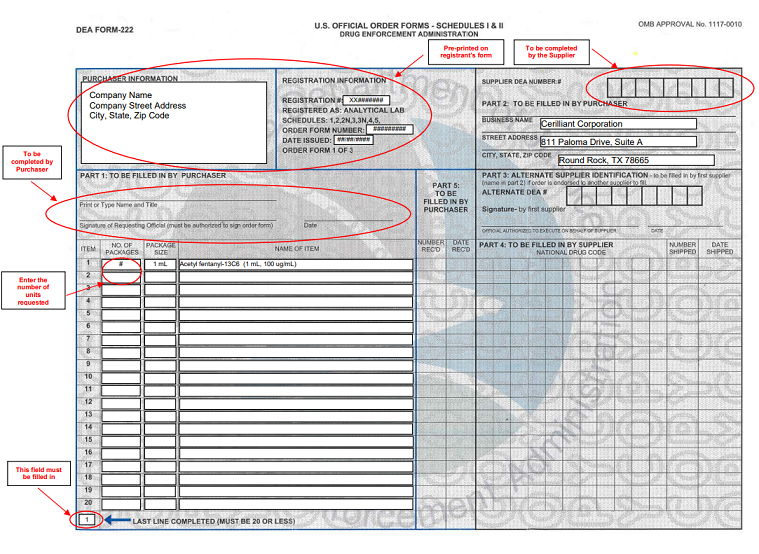
How To Fill Out Dea Form 222 - Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is. Instructions for filling out the new dea 222 form for pharmacy returns to the wholesaler of cii c2 controlled drug medications. § 1305.13 procedure for filling dea forms 222. Number of items and date received: (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. Once the order is received, complete the number of items and date they were received on your photocopy.
222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is. Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. Number of items and date received: Once the order is received, complete the number of items and date they were received on your photocopy. § 1305.13 procedure for filling dea forms 222. Instructions for filling out the new dea 222 form for pharmacy returns to the wholesaler of cii c2 controlled drug medications. (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the.
Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. § 1305.13 procedure for filling dea forms 222. Instructions for filling out the new dea 222 form for pharmacy returns to the wholesaler of cii c2 controlled drug medications. Number of items and date received: (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. Once the order is received, complete the number of items and date they were received on your photocopy. 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is.
RECORD OF DEA 222 USE Marquette University Doc Template pdfFiller
(a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is. Once the order is received, complete the number of items and.
Fillable Online Using DEA Form 222 to Order Controlled Substances Fax
222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is. Number of items and date received: Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. Instructions for.
DEA222 Form Instructions MedVet International
§ 1305.13 procedure for filling dea forms 222. Once the order is received, complete the number of items and date they were received on your photocopy. (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. Instructions for filling out the new dea 222 form for pharmacy returns to the.
Fillable Online DEA 222 Form Instructions for Schedule I & II
§ 1305.13 procedure for filling dea forms 222. Number of items and date received: (a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. Instructions for filling.
DEA form 222 A Guide to the Rules and Usage
(a) a purchaser must make a copy of the original dea form 222 for its records and then submit the. 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is. § 1305.13 procedure for filling dea forms 222. Instructions for filling.
How to Check DEA & Utilize Form 222 YouTube
Number of items and date received: 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is. § 1305.13 procedure for filling dea forms 222. Once the order is received, complete the number of items and date they were received on your.
Cerilliant Certified Reference Materials Certified Reference Standards
Number of items and date received: § 1305.13 procedure for filling dea forms 222. Once the order is received, complete the number of items and date they were received on your photocopy. Instructions for filling out the new dea 222 form for pharmacy returns to the wholesaler of cii c2 controlled drug medications. (a) a purchaser must make a copy.
PPT Chapter 2 PowerPoint Presentation ID250015
§ 1305.13 procedure for filling dea forms 222. Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is..
Single Sheet Dea 222 Form Instructions at Bobby Mosca blog
Once the order is received, complete the number of items and date they were received on your photocopy. Number of items and date received: 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is. Under 21 cfr 1305.03, the completion of.
Fillable Online DEA 222 Form Single Sheet Instructions mmscms
Instructions for filling out the new dea 222 form for pharmacy returns to the wholesaler of cii c2 controlled drug medications. 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is. (a) a purchaser must make a copy of the original.
§ 1305.13 Procedure For Filling Dea Forms 222.
Number of items and date received: Instructions for filling out the new dea 222 form for pharmacy returns to the wholesaler of cii c2 controlled drug medications. Once the order is received, complete the number of items and date they were received on your photocopy. 222 form orders that cannot be filled due to product availability will be held up to 60 days from form date so order can be filled when product is.
(A) A Purchaser Must Make A Copy Of The Original Dea Form 222 For Its Records And Then Submit The.
Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance.